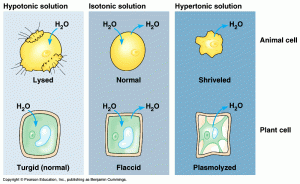
HYPERTONIC
A hypertonic solution is one with a high concentration of solutes when compared to another solution which is separated from it by a semipermeable membrane . The property of tonicity is often used to illustrate the biology of the body, with the solute concentration of cells and surrounding fluids being used as an example. Tonicity is related to osmosis , in which fluids flow back and forth across a semipermeable membrane; osmolarity differs from tonicity in that it considers the concentration of solutes that penetrate the membrane and those that do not, while tonicity only considers those that do not penetrate.
If a solution is hypertonic, it means that fluid will flow across the membrane and into the hypertonic solution until an isotonic state is reached. In an isotonic state, the solutions on either side of the membrane have the same distribution of solutes. Conversely, with a hypotonic solution, the concentration of solutes is lower than that of a solution on the other side of a membrane, which means that water will be drawn out of the hypotonic solution and into a hypertonic solution.
ISOTONIC
In the general sense, two solutions are isotonic when they contain the same amounts of solutes, or dissolved substances, and therefore have the same osmotic pressure. As commonly used in the medical field, though, isotonic solutions are solutions which have the same concentration of solute as the cells in the human body. A cell placed in an isotonic solution will neither gain nor lose water.
When two aqueous solutions of different concentrations or tonicities are separated by a semi-permeable membrane such as a cell wall, water will migrate from the less concentrated, or hypotonic, side to the more concentrated, or hypertonic, side in an attempt to bring both sides into equilibrium. This process is known as osmosis. The greater the difference in the two solutions’ concentrations, the higher the osmotic pressure will be, and the quicker the osmotic transfer will be.
|
HYPOTONIC
A solution which has a lower osmotic concentration (high water potential) than another solution is said to be hypotonic. If two solutions are of equal concentration they are isotonic.
A plant cell behaves differently from an animal cell when placed in a hypotonic solution. Since the cell sap has a lower water potential than that of the solution outside the living cell, water enters the cell by osmosis (endosmosis). Note, that the partially permeable membrane here is the plasma membrane and not the cellulose cell wall. The cellulose cell wallis permeable and allows most dissolved substances to pass through.
As water enters the cell the vacuole increases in size and pushes the cell contents against the cellulose wall. The plant cell does not burst because the cell wall is strong and relatively inelastic. It prevents over expansion of the cell by exerting an opposing pressure preventing the entry of more water. When the cell is in this state, it becomes rigid or turgid. This rigidity of the cell with water is called turgor. The pressure exerted by the water on the cell wall is the turgor pressure.
On the other hand an animal cell will swell and may burst in a hypotonic solution.
 |